
New atoms are also naturally produced on Earth as radiogenic daughter isotopes of ongoing radioactive decay processes such as alpha decay, beta decay, spontaneous fission, cluster decay, and other rarer modes of decay. On Earth, small amounts of new atoms are naturally produced in nucleogenic reactions, or in cosmogenic processes, such as cosmic ray spallation. Almost all other elements found in nature were made by various natural methods of nucleosynthesis. The lightest chemical elements are hydrogen and helium, both created by Big Bang nucleosynthesis during the first 20 minutes of the universe in a ratio of around 3:1 by mass (or 12:1 by number of atoms), along with tiny traces of the next two elements, lithium and beryllium. The discovery and synthesis of further new elements is an ongoing area of scientific study. Save for unstable radioactive elements ( radionuclides) which decay quickly, nearly all of the elements are available industrially in varying amounts. The first 94 occur naturally on Earth, and the remaining 24 are synthetic elements produced in nuclear reactions. The periodic table summarizes various properties of the elements, allowing chemists to derive relationships between them and to make predictions about compounds and potential new ones.īy November 2016, the International Union of Pure and Applied Chemistry had recognized a total of 118 elements. This table organizes the elements by increasing atomic number into rows (" periods") in which the columns (" groups") share recurring ("periodic") physical and chemical properties. Much of the modern understanding of elements developed from the work of Dmitri Mendeleev, a Russian chemist who published the first recognizable periodic table in 1869. Attempts to classify materials such as these resulted in the concepts of classical elements, alchemy, and various similar theories throughout human history. The history of the discovery and use of the elements began with primitive human societies that discovered native minerals like carbon, sulfur, copper and gold (though the concept of a chemical element was not yet understood). Air is primarily a mixture of the elements nitrogen, oxygen, and argon, though it does contain compounds including carbon dioxide and water. Nearly all other naturally occurring elements occur in the Earth as compounds or mixtures. Only a minority of elements, such as silver and gold, are found uncombined as relatively pure native element minerals. When different elements undergo chemical reactions, atoms are rearranged into new compounds held together by chemical bonds. This is in contrast to chemical compounds and mixtures, which contain atoms with more than one atomic number.Īlmost all of the baryonic matter of the universe is composed of chemical elements (among rare exceptions are neutron stars). For example, oxygen has an atomic number of 8, meaning that each oxygen atom has 8 protons in its nucleus. The basic particle that constitutes a chemical element is the atom, and each chemical element is distinguished by the number of protons in the nuclei of its atoms, known as its atomic number. Hence its atomic number is 20 and atomic mass number is 20 + 20 = 40.A chemical element is a chemical substance that cannot be broken down into other substances. For calcium, the number of protons and neutrons is equal i.e. Note: Atomic number is the number of protons present in the nucleus and atomic mass number is the sum of protons and neutrons present in the nucleus of an atom. Elements in the modern periodic table are arranged in increasing order of their atomic number. Modern form of the periodic table is based on atomic numbers. Hence, atomic number is always determined by the number of protons present in the nucleus of an atom. Similarly, it is not always necessary that the number of neutrons will be the same as the number of protons.Īs in the case of isotopes, the number of neutrons changes but the number of protons always remains constant.

Hence, the number of electrons in an atom are not fixed always. We know that electrons can be gained or lost during chemical reactions. the nucleus of the Calcium atom contains 20 protons. The total number of protons present in the nucleus of an atom gives the atomic number of that particular element.Ītomic number of Calcium is 20 i.e. We all know that protons, neutrons and electrons together constitute an atom. Similarly, the number of protons and neutrons are also associated with atomic number.
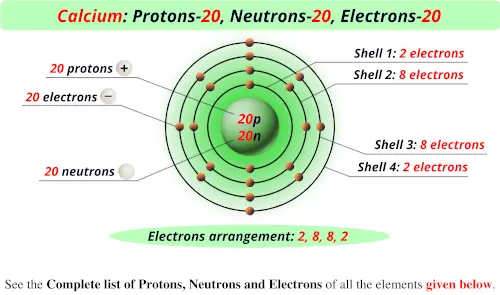
The number electrons depends on the atomic number. Hint: We determine the valency of an atom by knowing the number of electrons present in its outermost shell.


 0 kommentar(er)
0 kommentar(er)
